High-Performance Barrier Laminates for Reusable Gowns and Drapes
Surgical and Non-Surgical Gowns
Selecting the best level of protection for the standard ANSI/AAMI PB70:2012 involves understanding the critical zones of a gown and what each barrier performance level entails so that the best gown is chosen for use by healthcare workers. The critical zones of gowns comprise the front of the gown and the sleeves, both primary areas with the greatest risk of exposure to fluids and blood-borne pathogens. As the level increases, so does the need for greater barrier protection for the entire critical zone.
|
|
 |
 |
 |
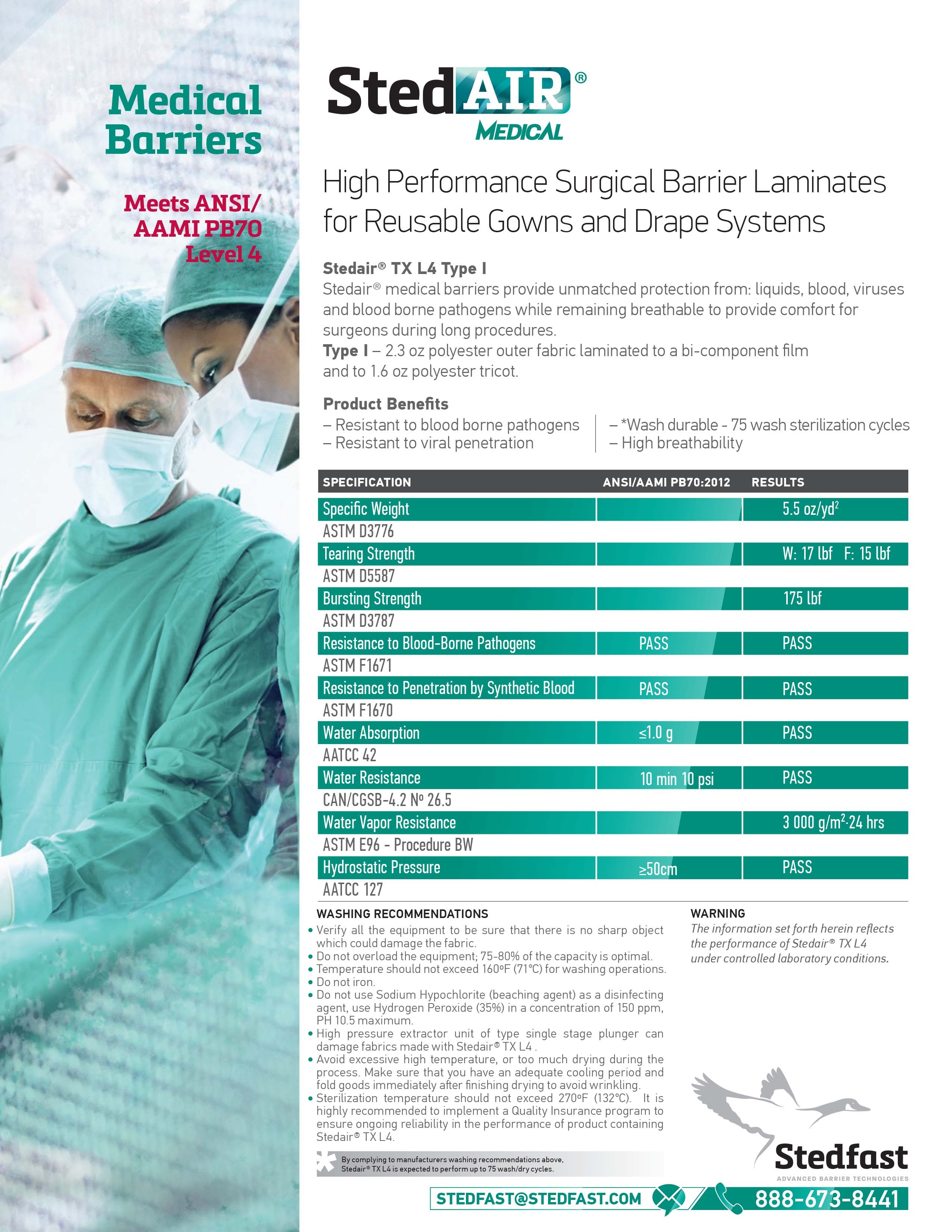
Stedair® TX L4 Level I |
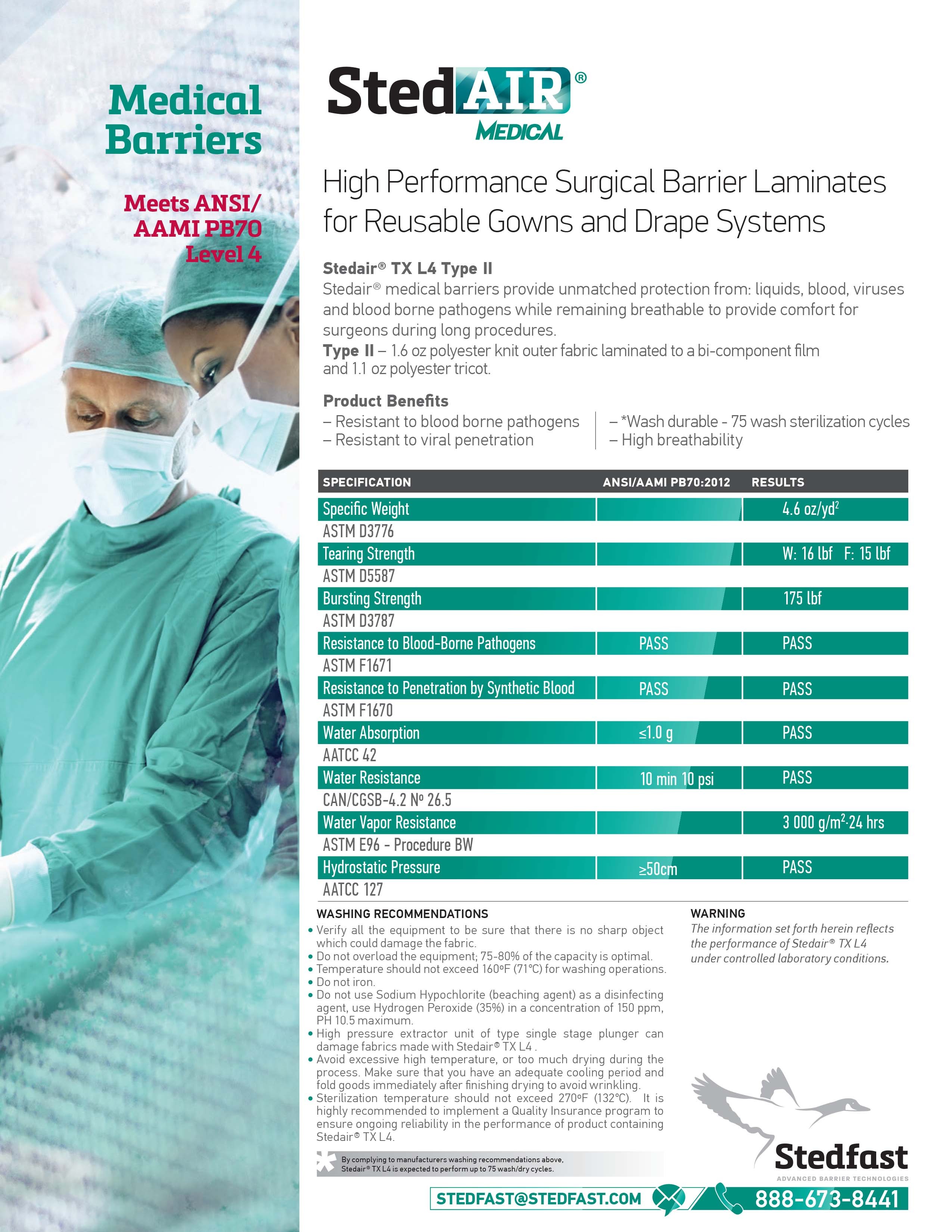
Stedair® TX L4 Level II |
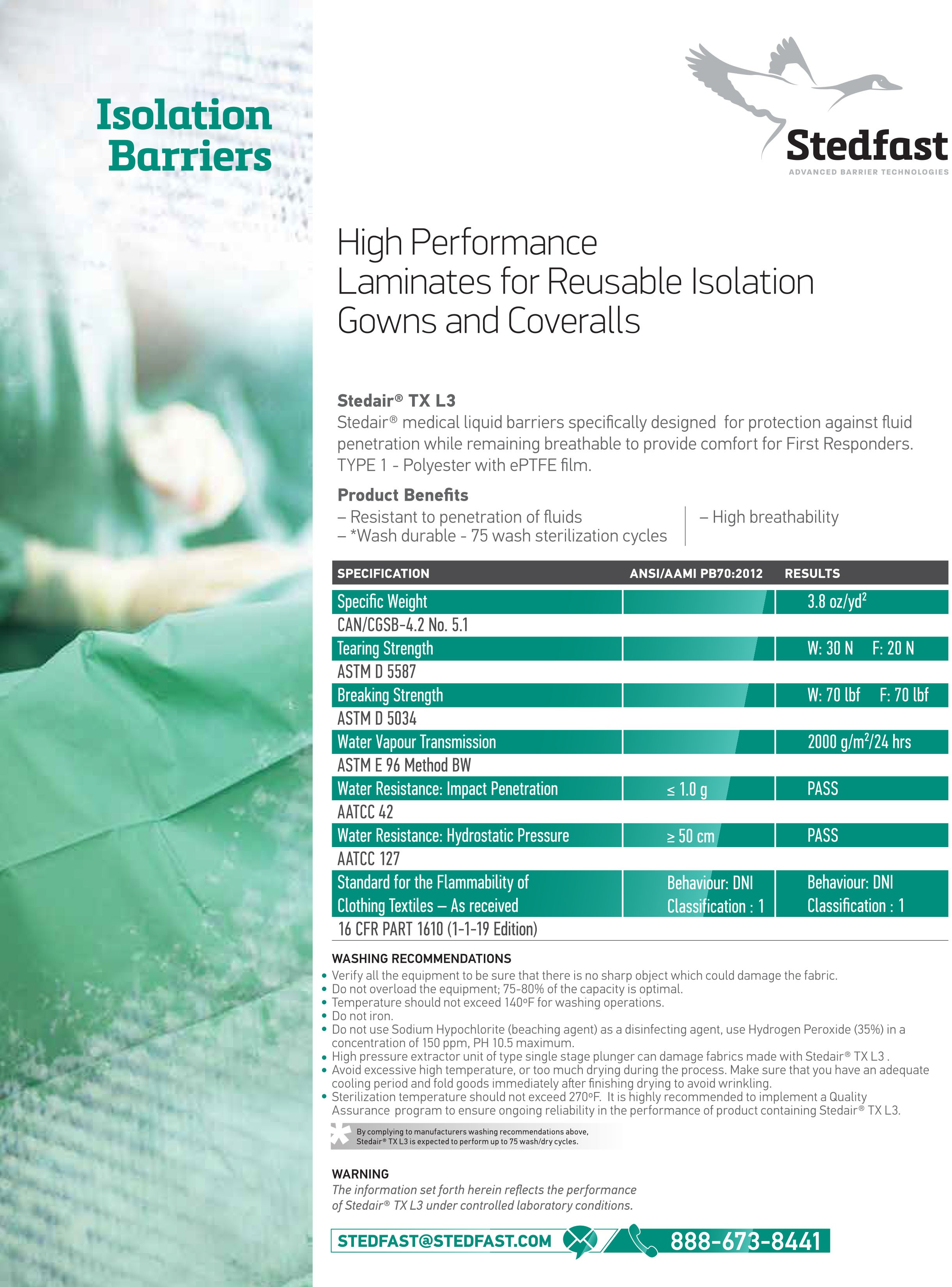
Stedair® TX L3 Isolation |
Stedair® TX L3 - Isolation Barriers
Stedair® isolation barriers are specially designed to protect against fluid penetration while remaining breathable to ensure the comfort of first responders. Non-surgical gowns are designed to protect the wearer against the transfer of micro-organisms and body fluids in low- and minimal-risk situations.
- Resistant to fluid penetration
- Durable washability
- Highly breathable
To Attain Level 3 Classification:
The hydrostatic pressure test measures the resistance of the critical zones to liquid penetration. The level 3 material must have a result greater than 50cm.
The water resistance measures the resistance of fabrics to water penetration by impact. The level 3 material must have a result of less than 1.0 grams.
| Test | Result |
| AATCC 42 Water Resistance | ≤ 1.0g |
| AATCC 127 Hydrostatic Pressure | ≥50cm |
Stedair® TX L4 - Level 4
Stedair® medical barriers offer unmatched protection against fluids, blood, viruses, and blood-borne pathogens while remaining breathable to keep surgeons comfortable during lengthy procedures.
To Attain Level 4 Classification:
The material must be subjected to the blood barrier test (ASTM F1670) and the viral barrier test (ASTM F1671). The blood barrier test measures a material’s resistance to synthetic blood under constant contact. The sample is mounted on a cell between the synthetic blood and a viewing port, then subjected to atmospheric pressure for 5 minutes, 2.0 psi for 1 minute and atmospheric pressure for 54 minutes. If there is any strikethrough on the sample during the 60-minute test, the sample fails the test. The viral barrier test measures the material’s resistance to penetration of a microorganism under constant contact. The sample is mounted similarly to the blood barrier test and is then subjected to the same time and pressure protocol. If any liquid penetration occurs, the sample fails. If there is no visible liquid penetration at the end of 60 minutes, a microbial assay is performed to determine if any non-visible penetration occurred. If any microbial penetration is found, the sample fails the test.
| Test | Result |
| ASTM F1671 - Resistance to blood-borne pathogens | PASS |
| ASTM F1670 - Resistance to penetration by synthetic blood | PASS
|
Purchase From our Partners
 ventes_sales@logistikunicorp.com |
 |
 |
 |
 |
|
 |

